
Fullerene C60 is sometimes seen as a big molecule, sometimes as a small hollow cluster, sometimes as a graphene sheet wrapped up to a spherical shape. Strong C-C σ-bonding stabilize a rigid backbone structure. Substantially delocalized π electrons account for the chemical and optical properties of C60. Below 800 K, C60 forms a Van der Waals insulating solid of loosely bound individual molecules, which rotate almost freely down to approximately 260 K. Intercalation of alkali elements in C60 can form ionic materials (fullerides), showing novel and exciting properties such as superconductivity, organic magnetism, correlated Mott-insulating states.

The hole wave function in one of the 5 degenerate HOMO orbitals.
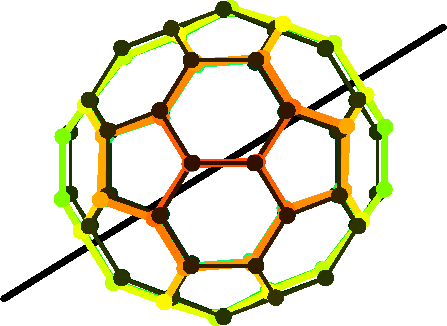
The distortion of the cation C60+ (coloured) with respect to the uncharged ideal icosahedral configuration. The distortion is amplified by a factor 10 to make it visible.
A colorful picture of C60. Distance modulates the color.